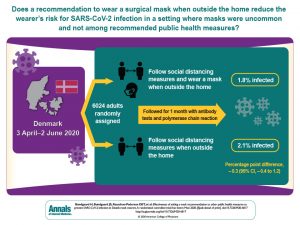
“Predicting the binding mode of flexible polypeptides to proteins is an important task that falls outside the domain of applicability of most small molecule and protein−protein docking tools. here, we test the small molecule flexible ligand docking program glide on a set of 19 non-α-helical peptides and systematically improve pose prediction accuracy by enhancing glide sampling for flexible polypeptides. in addition, scoring of the poses was improved by post-processing with physics-based implicit solvent mm- gbsa calculations. using the best rmsd among the top 10 scoring poses as a metric, the success rate (rmsd ≤ 2.0 å for the interface backbone atoms) increased from 21% with default glide sp settings to 58% with the enhanced peptide sampling and scoring protocol in the case of redocking to the native protein structure. this approaches the accuracy of the recently developed rosetta flexpepdock method (63% success for these 19 peptides) while being over 100 times faster. cross-docking was performed for a subset of cases where an unbound receptor structure was available, and in that case, 40% of peptides were docked successfully. we analyze the results and find that the optimized polypeptide protocol is most accurate for extended peptides of limited size and number of formal charges, defining a domain of applicability for this approach.”